Accidental poisoning. In emergency situations, doctors need to find out fast which substances were ingested. So, makers of hazardous chemicals are required to submit product details to the relevant agencies. Now companies can save time and resources by streamlining and automating their PCN compliance process.
Understanding the Poison Centre Notification regulation
If you are a manufacturer or importer of hazardous mixtures in the EU, you are aware that you have to comply with the Poison Centre Notification (PCN) regulation (Annex VIII to CLP).
The EU PCN is intended to standardize information across Europe relating to hazardous chemicals or mixtures that are hazardous. It ensures that manufacturers, importers and downstream users notify poison centers about the specific substances in consumer products.
This means that you have to submit a PCN dossier to the European Chemicals Agency (ECHA) for each mixture classified as hazardous to human health that you place on the market. You must also update it whenever there is a change in your formulation, packaging, legal entity or other relevant information.
Submission requirements of the PCN
Under the EU PCN, companies must submit the concentration range for components of major concern that present a risk to health. These include, for example, substances and mixture components that could cause skin corrosion, serious eye damage or are acutely toxic. Organizations must also disclose specific nonhazardous mixture components when they are present above certain concentrations.
The consequences of noncompliance can include fines and penalties. But keeping track of changes and updating your PCN dossiers on time can be a challenging and time-consuming task. The challenges and problems include the difficulty in correctly identifying all applicable changes, as well as spending time and resources to support this analysis that could be better utilized for other business needs.
Complying with the EU PCN
When your organization has submitted the necessary PCN dossiers, the work is not over. You must keep your submissions up to date. Companies have to monitor and disclose any changes in:
- Substances and intermediates.
- Mixtures in mixtures (MiM).
- Interchangeable component groups (ICGs).
- Market placements or packaging of your mixtures.
Following significant changes in the composition of your mixtures, you also have to notify the departments responsible for updating the labels with the new unique formula identifier (UFI). And you have to do all this without undue delays and before placing the changed product on the market, as required by ECHA.
The timeline for submissions depends upon how your product will be used. If materials supplied to an industrial site are eventually used in consumer products, information on these must also be submitted.
PCN automation: How Sphera can help
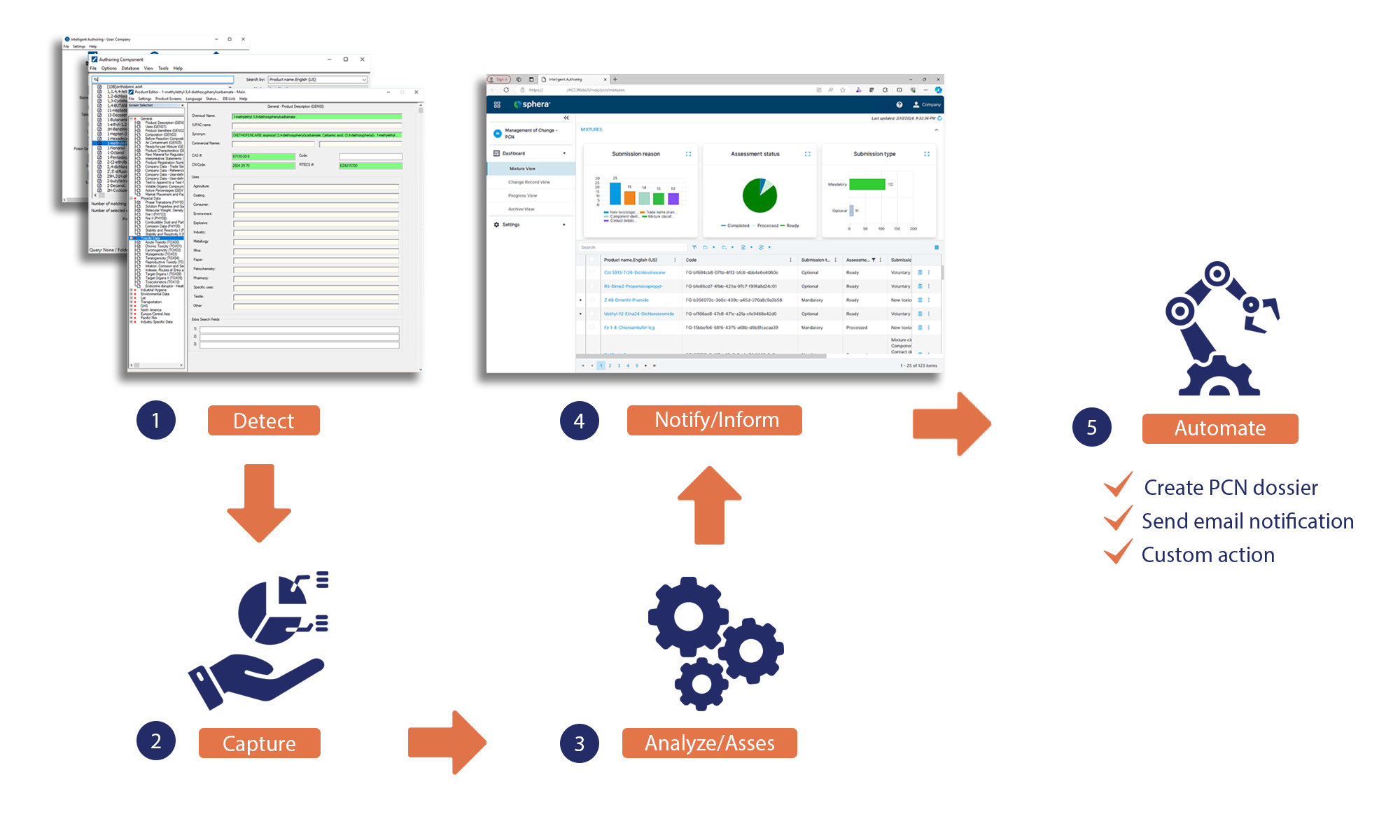
At Sphera, we know how challenging it is to keep up with all the changes and to update your PCN dossiers in a timely manner. That is why we are excited to introduce our new product offering: Management of Change for PCN. Here is how our solution helps you automate your PCN compliance process to save time and resources:
Detect and identify: Management of Change for PCN detects any changes in the components of your mixtures that have the potential to impact your PCN dossiers. It captures the changes and identifies which mixtures with active PCN dossiers are affected.
Analyze and assess: Our solution analyzes and assesses the impact of the changes on your PCN dossiers and determines whether they require a mandatory or optional submission update. When the Intelligent Authoring settings are activated, Management of Change for PCN also generates a new UFI for your mixture.
Visualize and inform: Through a user-friendly dashboard, Management of Change for PCN notifies you of the changes and their impact. You can see which mixtures need to be updated, the type of change and the submission type (mandatory or optional).
Automate and notify: Sphera’s new offering also enables further automation, such as automatically creating a new PCN dossier for changes that require a mandatory update. It can also send email notifications to the departments responsible for updating the labels with the new UFI.
With Management of Change for PCN, you can streamline your PCN compliance process and ensure that you submit relevant updates to your PCN dossier on time. You can also avoid errors, fines and delays that may result from noncompliance. And you can focus on your core business activities while we take care of your PCN obligations.
If you are interested in learning more about Management of Change for PCN, please contact us today. We would be happy to show you a demo and discuss how we can customize our solution to meet your specific needs.

