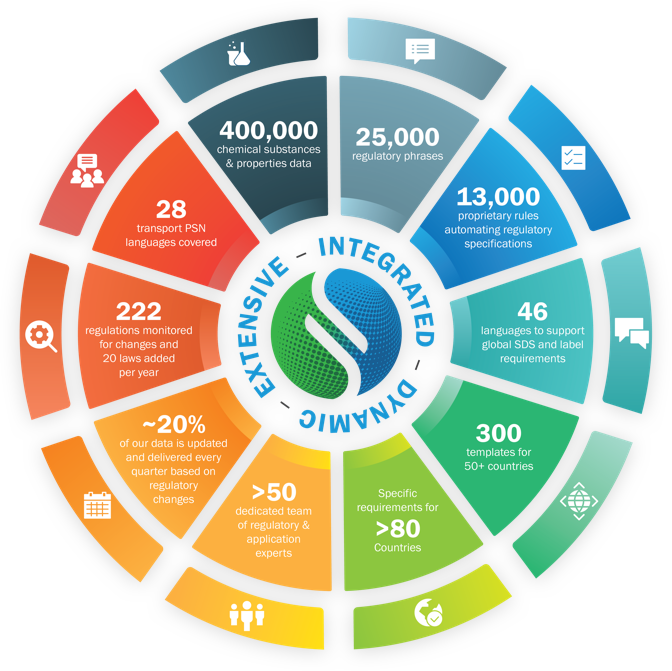
Not all content is created equal.
When you’re navigating the complexities of global chemical regulations, the right combination of technology and managed regulatory content is critical to maintaining compliance. With Sphera’s authoring solutions, industry-leading content is at your fingertips so you can comply with confidence.
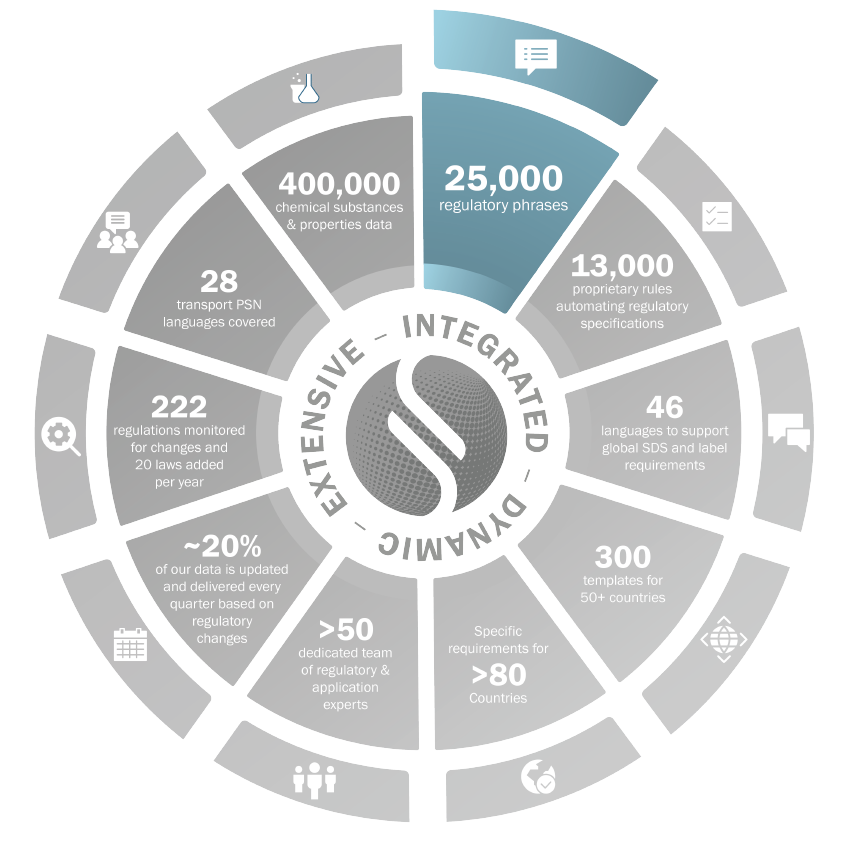
Phrases
Labeling phrases from primary regulatory sources
Non-regulatory phrases translated and peer reviewed by domain experts
Exposure Scenarios phrases from ESCOM standard phrase Catalogue
25,000+ phrases for authoring
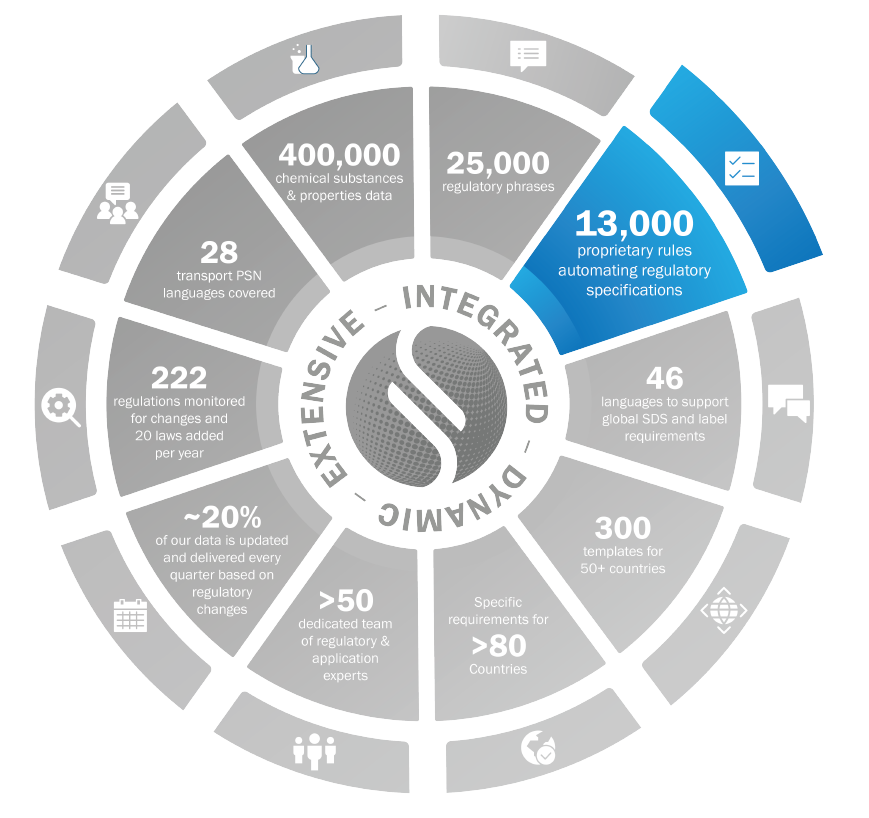
Rules
Audits provide traceability of the inputs considered for supporting an output
Open source grants endless capabilities in tailoring existing or creating new rules
Fast execution enabling the authoring of a coherent and compliant SDS in seconds
13,000+ document authoring
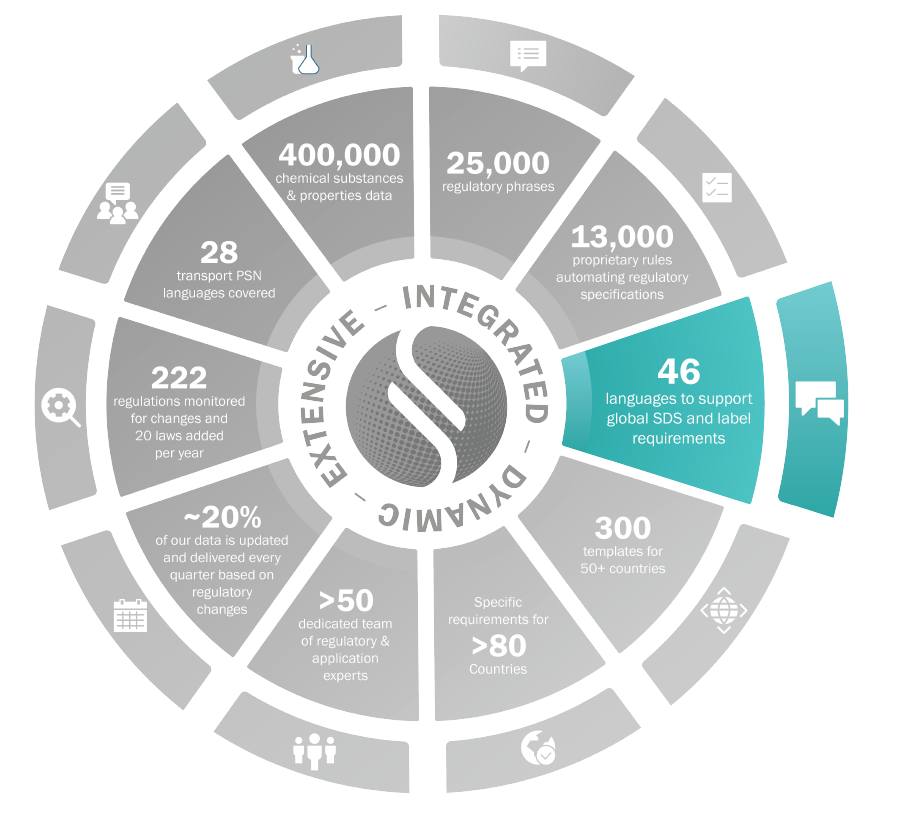
Languages
Translated chemical names provided for more than 50,000 substances
Supports double-byte character sets (Chinese, Japanese, Korean), and right to left languages (Hebrew, Arabic…)
American and European dialects of English, Spanish, French and Portuguese
46 languages translated
Templates
Template editor for users to tailor existing documents, or create their own (Reports, Product Declarations, Workplace Safety Cards…)
SDS and label templates designed and maintained to comply with country specific regulations
300+ templates provided in the solution
Countries
Occupational Exposures Limits for more than 50 countries and all member states of the European Union
GHS Specific implementation for more than 30 jurisdictions, and expandable with GHS by design
80+ requirements made for countries
Team
50 full time employees dedicated to the maintenance, improvement and support of managed regulatory content with customers engaged during the design, development and beta test cycles
Regulatory experts, programmers, toxicologists, authors, customer service, product managers, project managers, maintaining, improving and supporting our offerings
50 full time employees
Monitoring
Communication for important regulatory changes
Changed lists are updated and delivered, thus ensuring continuous compliance
Manual and automated monitoring. Interaction with government agencies
20% updated and delivered quarterly
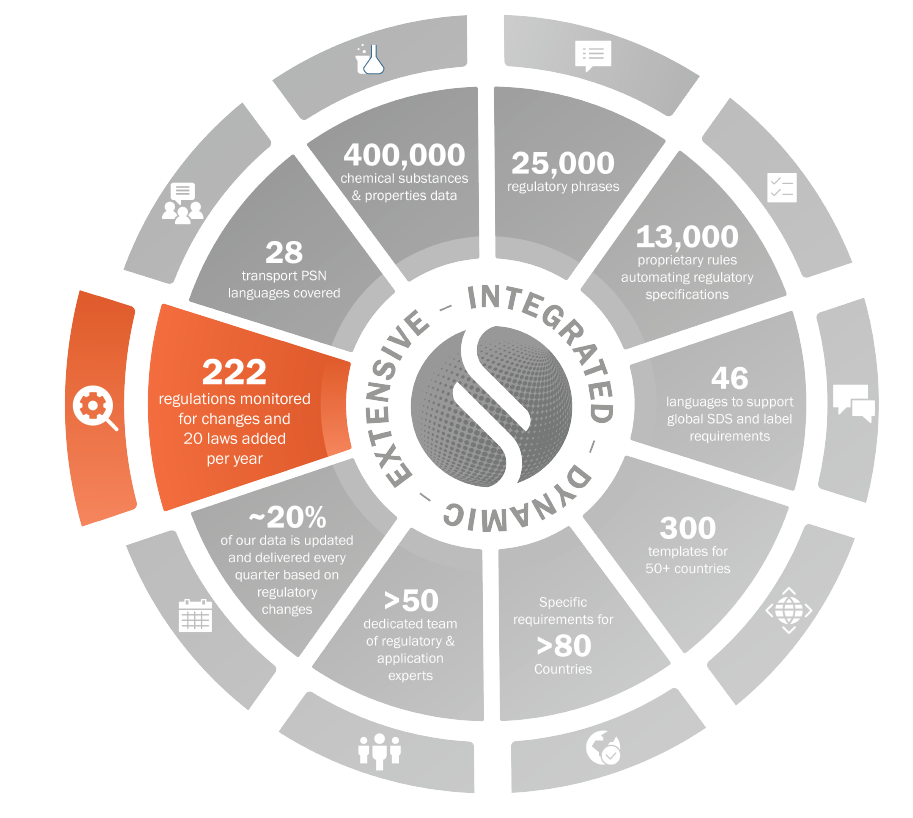
Regulations
Hazard communication
- Classification of substances and mixtures
- “Verbiage” (first-aid, handling, storage)
Industry Specific
- Calculation of VOC net content in mixtures
- CEPE Guidelines (Paints & coatings)
- IFRA Certificates (Flavors and fragrances)
Chemical control
- National Inventory lists
- Bans/restrictions
222 regulations monitored
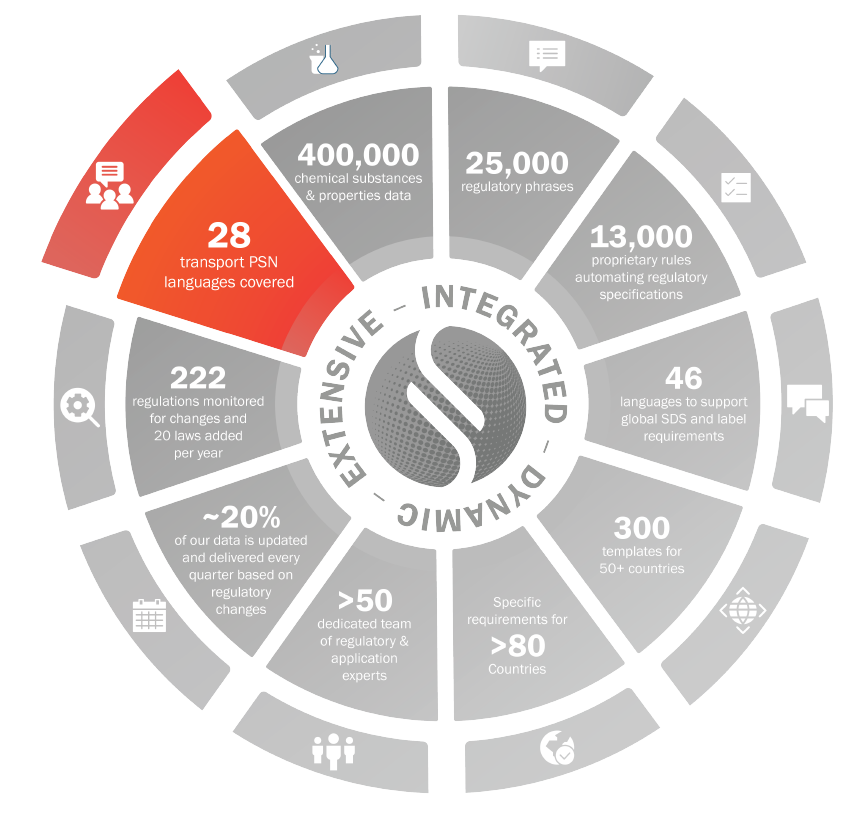
Transport
Automated transport classification for mixtures, authoring section 14 of an SDS
Specific coverage for IATA, IMDG, DOT, TDG, ADG, NOM Standards etc…
Translated Proper Shipping Names
28 PSN languages covered
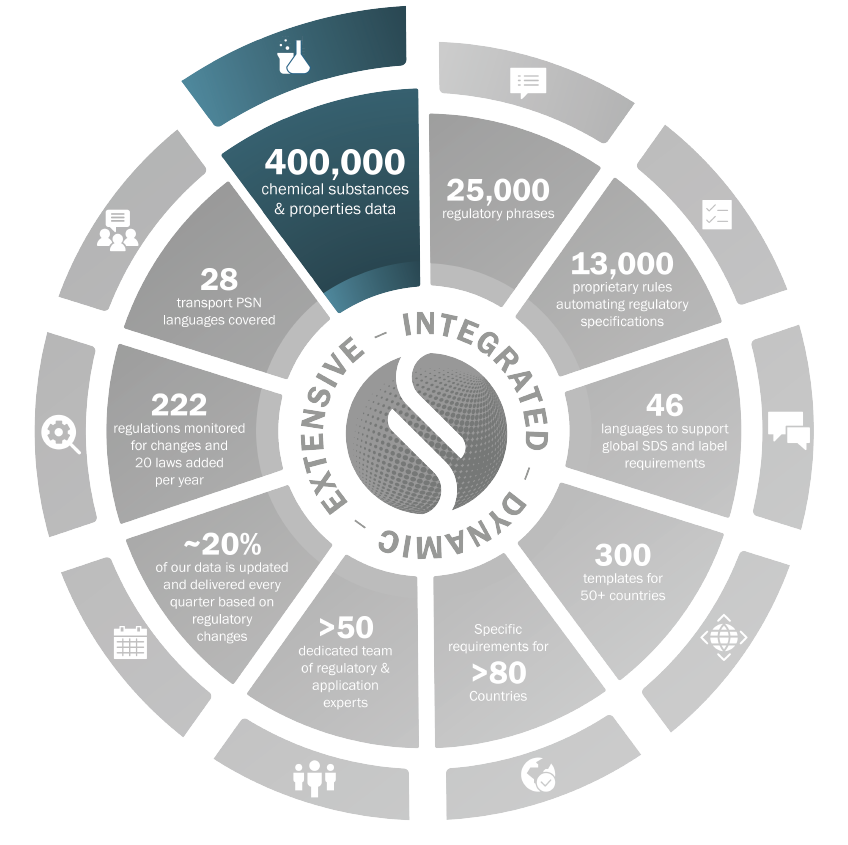
Substance Data
Data from registered substances as per REACH (ECHA Source)
Toxicity Data from RTECS, Ecotoxicity Data from EPA AQUIRE
All substances from all National Inventories. Sphera Experts associate substances to Group Definitions
400,000+ chemical substances & properties data
Related Content
Engage with our robust Product Stewardship content library.
Taking Total Control: 4 Reasons Your Business Needs Comprehensive Product Stewardship
Episode 41: This Podcast Is About Control, Total Control
.
EU Poison Centre Notification (PCN) E-Book
.
Ready to learn more?
Tell us how we can help.